# Clinical Evidences
The Clinical Evidence page in SEQ Platform provides a centralized, curated resource connecting detected biomarkers to relevant therapies, clinical guidelines, and trial data. It enables users to quickly interpret somatic findings in a clinically meaningful context by integrating data from NCCN, FDA, and ongoing clinical trials.
Each matched evidence entry is displayed in an interactive table that supports sorting, searching, and filtering.
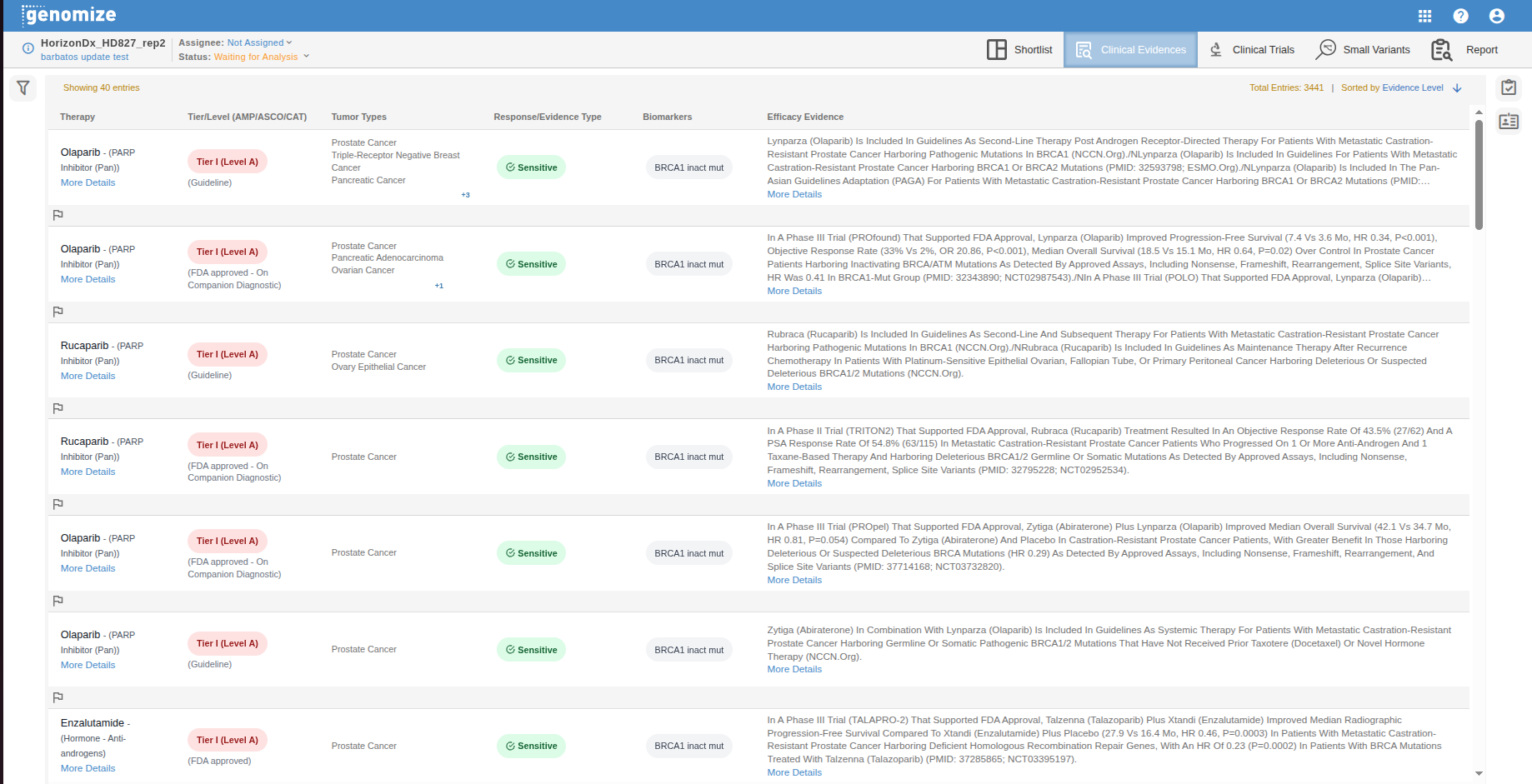
Therapy: The drug or therapeutic agent (e.g., Cetuximab, Panitumumab) associated with the detected molecular profile.
Tier/Level and Approval Status: The AMP/ASCO/CAP Tier/Level classification and regulatory status describing the strength of the evidence (e.g., Guideline, Phase I, FDA Approved).
Tumor Types: The cancer type(s) for which the evidence is applicable (e.g., Colorectal Cancer, Acute Myeloid Leukemia).
Response/Evidence Type: The observed or predicted therapy response (e.g., Sensitive, Resistant, Predicted – Sensitive).
Biomarkers: The detected variant(s) or molecular alteration(s) that triggered the evidence match (e.g., BRAF V600E).
Efficacy Evidence: A concise summary of clinical findings and their supporting sources (e.g., NCCN.org, ESMO.org).
Molecular Profile Details: Clicking a row expands a detailed subview showing the exact variant(s) or biomarker(s) from the current sample that matched the evidence. This view allows users to confirm whether the patient’s molecular data meet the evidence’s eligibility criteria.
# Filtering Options
SEQ Platform allows users to dynamically filter the evidence table to focus on clinically relevant findings.
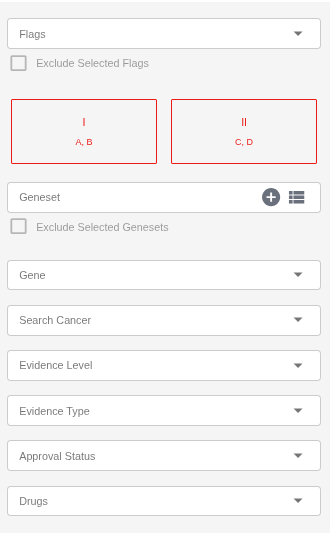
Flags: Filter evidence based on custom user-defined flags (e.g., Include in Shortlist).
Geneset: You can create your own geneset using the plus icon in this field and use it in your filters. You can create as many genesets as needed. You can also use system-defined genesets, which are updated weekly or upon the release of a new version — for example, the ACMG’s list of recommended genes for incidental findings.
Gene: Search for genes using the autocomplete feature.
Cancer: Search for cancers using the autocomplete feature.
Evidence Tier/Level: Filter results based on the AMP/ASCO/CAP Tier/Level classification (e.g., Level A, Level C, Tier I, Tier II).
Evidence Type: Filter by evidence category (e.g., Sensitive, Resistant, Prognostic, Diagnostic, etc.).
Approval Status: Filter by guideline or regulatory status (e.g., Guideline, FDA Approved).
Drugs: Search for evidence associated with specific therapies or compounds.